 Oivd Workshop Premarket Notification 510 K April 22 2003
Oivd Workshop Premarket Notification 510 K April 22 2003

 43 Ideas Wordperfect Templates Free All About Resume
43 Ideas Wordperfect Templates Free All About Resume
 Modifications To A Cleared Device Letter To File Or New 510
Modifications To A Cleared Device Letter To File Or New 510
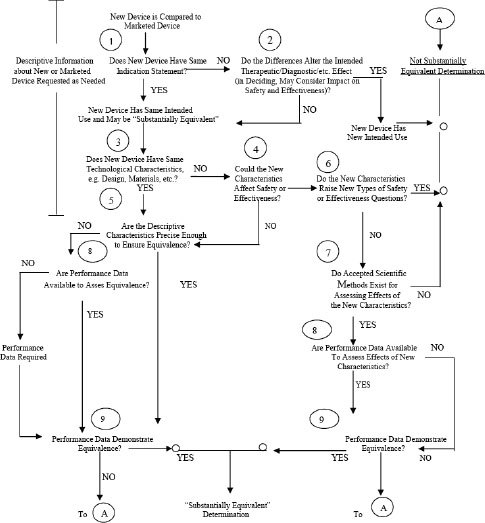 4 The 510 K Clearance Process Medical Devices And The
4 The 510 K Clearance Process Medical Devices And The
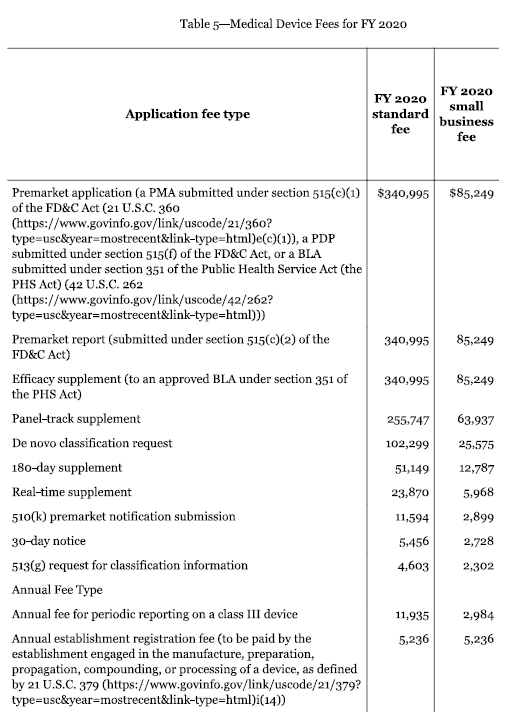 Medical Device 510k Submissions Quality Systems And
Medical Device 510k Submissions Quality Systems And
Indications For Use Case Study Medical Device Academy
 Fda 510 K Submission A Step By Step Guide On How To
Fda 510 K Submission A Step By Step Guide On How To
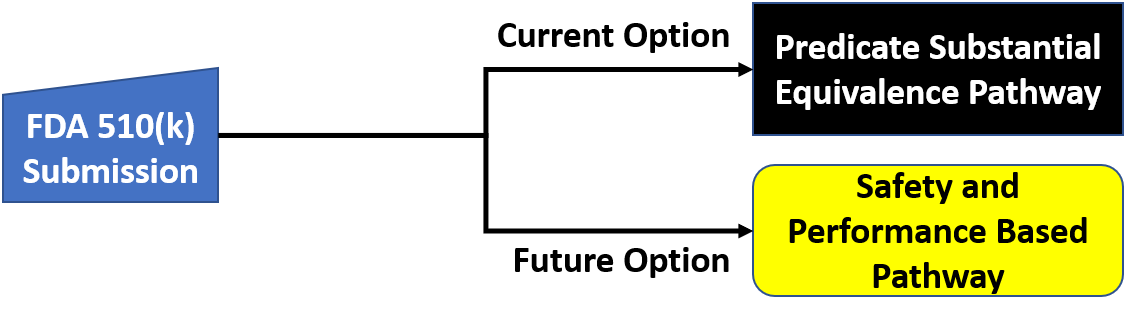 Us Fda Pre Market Notification 510 K Medical Device Academy
Us Fda Pre Market Notification 510 K Medical Device Academy
 College Admission Essay Writing Services Top 10 Tools For
College Admission Essay Writing Services Top 10 Tools For
 510k Cover Letter And Fda Form 3514 How To Webinar
510k Cover Letter And Fda Form 3514 How To Webinar
510k Premarket Notification Cdr



